Titan® Penile Implants
Performance from the operating room to the
bedroom

Coloplast Titan Implant
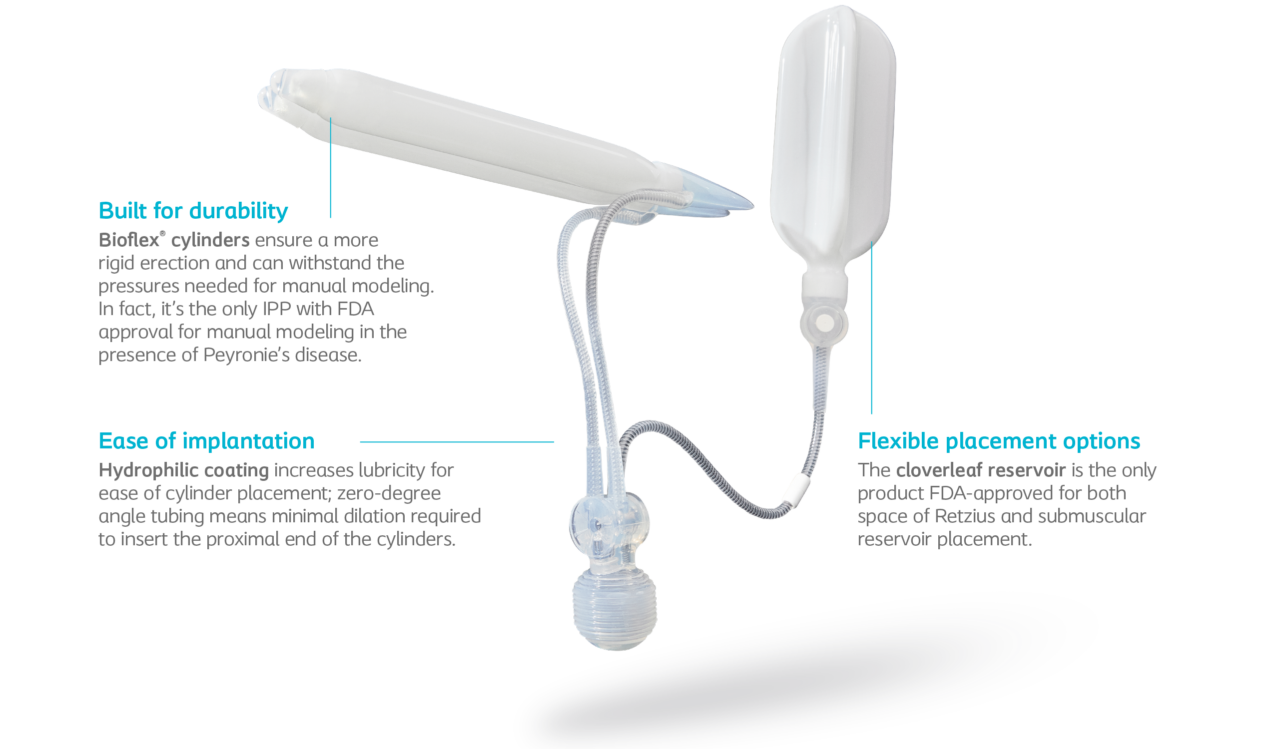
Built to perform.
Built to satisfy.
Titan implant provides performance in both the operating room and the bedroom. Titan implant is designed to overcome complications, address patient satisfaction, and allows you to serve the diverse needs of more patients that walk through your door.
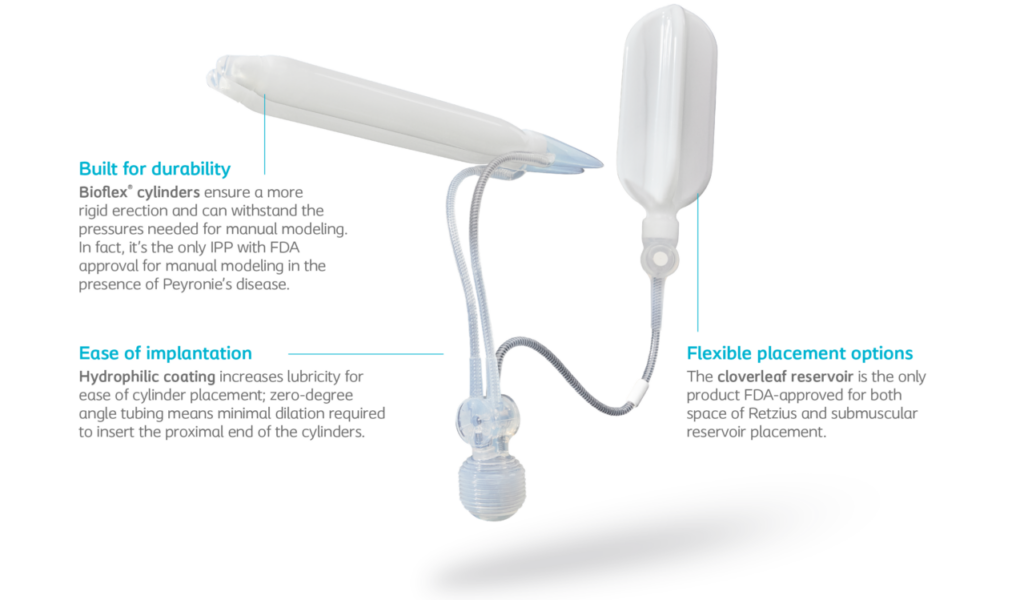
Titan implant performance in the operating room
Technology that’s designed and engineered for performance

Power of choice
HydroVANTAGE™ hydrophilic coating absorbs aqueous solution of physician’s choice on submerging the device in the solution.
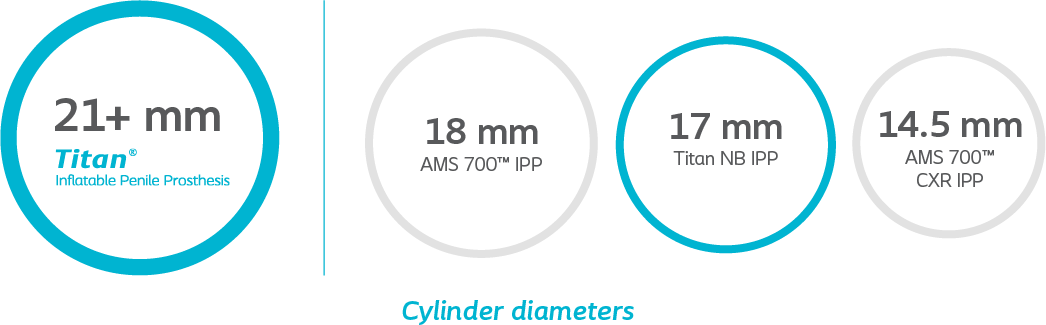
Tailored fit
Because there’s a wide variety in the male anatomy, Titan implant is available in a wide range of standard and narrow sizes so you can choose the right fit for each patient.
Cylinder diameters are not shown to scale.
*AMS 700™is a trademark of Boston Scientific Corporation.
Titan implant performance in the bedroom

Performance that satisfies so your patients can rediscover their confidence and get back to a healthy sex life
17%
MORE GIRTH1
compared to AMS 700™ CX Implant
Maximizing girth may lead to increased partner stimulation and satisfaction.
3x
MORE RIGID2
compared to AMS 700™ LGX Implant
Increased rigidity reduces the chance of buckling during sex.
3x
MORE DURABLE3
compared to AMS 700™ CX Implant
Enhanced penetration performance for more confidence in a variety of sexual positions so patients can feel uninhibited.
*AMS 700™is a trademark of Boston Scientific Corporation.
98%
Patient satisfaction with the Titan penile implant for erectile dysfunction4
96%
Patient satisfaction with the Titan penile implant for erectile dysfunction4
How does the Titan® implant work?
When you squeeze the pump in the scrotum, fluid moves from the reservoir into the cylinders in the shaft of the penis, creating an erection.
When you press the deflate button on the pump in the scrotum, the fluid moves out of the penis and back into the reservoir for a natural looking flaccid state.
View the following video for a demonstration.
No pills, no injections.
It’s all you.
The Titan implant is a long-term ED solution that puts you back in control of your intimacy.
Learn about Coloplast solutions
Titan & Titan Touch Inflatable Penile Prosthesis Brief Statement
Indications
The Titan Inflatable Penile Prosthesis is indicated for male patients with erectile dysfunction who are considered to be candidates for implantation of a penile prosthesis.
Contraindications
The Titan Inflatable Penile Prosthesis is contraindicated in patients who have one or more of the following conditions: Patients with an active infection present anywhere in the body, especially urinary tract or genital infection. Patients with a documented hypersensitivity or allergic reaction to silicone or polyurethane. Patients with unresolved problems affecting urination, such as an elevated residual urine volume secondary to bladder outlet obstruction or neurogenic bladder. Patients unwilling to undergo any further surgery for device revision.
Warnings
The Titan device should only be implanted by physicians experienced in the surgical procedures involving implantation of a penile prosthesis. Physicians should advise prospective patients, prior to surgery, of the warnings, precautions and potential complications associated with the use of this product, which may include the following: Potential for resurgery (Note: device is not a lifetime implant). Implantation makes latent natural erections, as well as other interventional treatment options, impossible. Implantation may result in penile shortening, curvature or scarring. Pre-existing abdominal or penile scarring or contracture may make surgical implantation more complicated or impractical. Diabetic, as well as immunocompromised patients, may have an increased risk of infection which could result in permanent damage to tissue/organs. Vigorous exercise and manual massage could lead to device damage. Certain stresses and pressures (straddle seating, obesity, etc.) could lead to involuntary inflation or deflation.
Precautions
Patients with spinal cord injury may have an increased risk of infection. This device may be used to treat ED in the presence of Peyronie’s disease. A thorough preoperative consultation should include a discussion between the patient and physician of available treatment options and their risks and benefits. Patient selection should consider the following factors which could lead to increased risk of failure and can be critical to the eventual success of the procedure: Ability and willingness of the patient to follow instructions. Associated psychological status (e.g. psychogenic erectile dysfunction, inappropriate attitude or motivation). Health conditions which hamper sexual activity, such as severe angina, may prevent successful use of this device. Manual dexterity problems.
Potential Complications
Adverse events are known to occur with penile protheses procedures and implants; some may require revision surgery or removal of the implant. Adverse events following penile protheses implantation may be de novo, persistent, worsening, transient, or permanent.
Adverse events may include but are not limited to: Acquired phimosis, Adhesion(s), Bladder storage symptoms, Capsular contracture, Deformity, Delayed/Impaired/Abnormal wound healing, Discomfort, Erosion/Extrusion, Fistula, Foreign body reaction, Hematoma/Seroma, Hemorrhage/Bleeding, Hernia, Hypersensitivity/Allergic reaction, Induration, Infection (local or systemic), Inflammation (including, but not limited to edema, erythema, redness, swelling), Male Dyspareunia, Necrosis, Obstruction/Occlusion, Pain, Perforation or injury of soft tissue (e.g., muscles, nerves, vessels), structures, or organs (e.g., bowel, bone, bladder, urethra, ureters), Scar tissue, Sexual dysfunction, Tactile disorders, e.g., hypoesthesia, numbness, Urinary incontinence symptoms, Urinary tract infection, Voiding symptoms.
The occurrence of these events may require one or more subsequent surgeries which may or may not always fully correct the complication.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-02124 / Feb 2024
References
- Pescatori ES, Goldstein I. Intraluminal device pressures in 3-piece inflatable penile prostheses: the pathophysiology of mechanical malfunction. J Urol. 1993 Feb;149(2):295-300.
- Scovell JM, Ge L, Barrera EV, Wilson SK, Carrion RE, Hakky TS. Longitudinal and Horizontal Load Testing of Inflatable Penile Implant Cylinders of Two Manufacturers: An Ex Vivo Demonstration of Inflated Rigidity. J Sex Med. 2016 Nov;13(11):1750-1757.
- Pritchard, Charles, MD, et al. “Comparison of AMS 700 CX and Coloplast Titan Inflatable Penile Prosthesis Cylinders Subjected to In-Vitro Cyclic Buckling.” Sexual Medicine Society of North America Fall Meeting, Poster 111, 2008.
- Garber BB. Mentor Alpha 1 inflatable penile prosthesis: patient satisfaction and device reliability. Urology. 1994 Feb;43(2):214-7.
PM-29356