Torosa® Testicular Implant
Empowering survivors beyond the standard

It’s more than cosmetic. For testicular cancer survivors, the physical and emotional trauma from the removal of a testicle may result in long-lasting feelings of loss, uneasiness or shame. Simply providing the option for a testicular prosthesis may help alleviate these effects and assist in their overall recovery.1
98%
believe it’s “important” to be offered one, whether they accept it or not2
50-60%
report improved body image after testicular prosthesis placement3
93%
of Torosa testicular prosthesis patients reported a high level of patient satisfaction in core clinical studies4
The implant you can trust for your patients
As the only FDA-approved testicular implant on the market, Torosa testicular prosthesis has been rigorously tested and clinically proven to be a safe and effective treatment for your patients.
Testicular prostheses are safe to implant at the time of orchiectomy for testicular cancer.5 Torosa can be implanted with few complications and with a low or absent risk of rheumatological disease.6
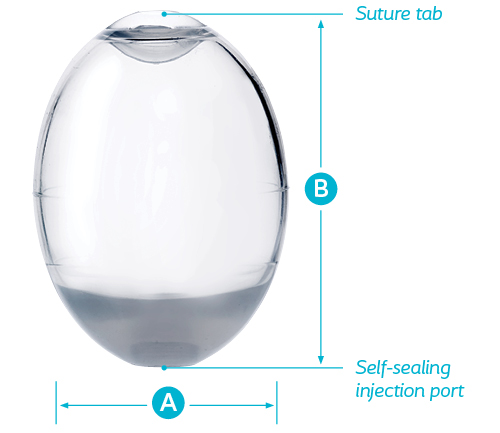
Features:
- Features molded silicone elastomer shell
- Available in 4 sizes to meet the needs of adult, adolescent, and pediatric patients
- Suture tab enables secure placement of the device in a set position, if desired, eliminating unwanted movement within the scrotum
- Self-sealing injection port allows for filling the device with sterile saline solution
Learn about Coloplast solutions
Torosa® Saline-Filled Testicular Prostheses Brief Statement
Indications
The Coloplast Torosa Saline-Filled Testicular Prosthesis is intended for use when cosmetic testicular replacement is indicated i.e., in the case of agenesis or following the surgical removal of a testicle.
Contraindications
The implantation of testicular prostheses is contraindicated in the presence of infection or untreated neoplasm.
Warnings
Implantation of a testicular prosthesis in patients with pre-existing varicoceles may result in persistent pain. Testicular implants should not be considered lifetime implants due to the inherent nature of silicone implants, implant procedures, and potential individual psychological reactions. This device contains solid silicone elastomer. The risks and benefits of implanting this device in patients with lupus (e.g., SLE or DLE), scleroderma (e.g., progressive systemic sclerosis), myasthenia gravis, or documented sensitivity to silicone should be carefully considered. The issue of the possible relationship between silicone and various diseases has been and continues to be the subject of scientific and medical debate. Sepsis, hemorrhage, thrombosis, or tissue necrosis may result from the placement of any foreign object in the body. Excessive fibrous capsular formation or contracture may occur around any implant placed in contact with soft tissues. The incidence and severity of this occurrence may increase if postoperative local hematoma or infection occurs.
Precautions
Implantation of the device may be difficult or impossible in patients with inadequate scrotal tissue to cover the prosthesis, patients who have undergone prior pelvic radiation therapy, or patients whose wound healing abilities are compromised (e.g., uncontrolled diabetes, poor circulation). Pre-existing infection should be treated and resolved before implantation of the prosthesis. These devices must only be used by surgically trained and experienced physicians. Saline-filled implants may leak over time and long-term results cannot be guaranteed. A thorough preoperative consultation should include a discussion between the patient and physician of all available treatment options and their risks and benefits.
Potential Complications
The following device and procedure-related adverse events were reported during the pivotal clinical trial: pain, discomfort, edema, extrusion, displacement/migration, genitalia hematoma, keloid formation, implant deflation, fluid accumulation (inguinal area), constipation, fibrosis, granuloma, mobile implant, neuropathy (leg), numbness (heel), and suture abscess.
Advice to Patient
Patients should be advised that testicular implants should not be considered lifetime implants due to the inherent nature of silicone implants, implant procedures and potential physiological reactions. It is the physician’s responsibility to advise the patient of all potential complications and risks associated with the use of testicular implants.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
References
- Skoogh J, Steineck G, Cavallin-Ståhl E, Wilderäng U, Håkansson UK, Johansson B, Stierner U; SWENOTECA. Feelings of loss and uneasiness or shame after removal of a testicle by orchidectomy: a population-based long-term follow-up of testicular cancer survivors. Int J Androl. 2011 Apr;34(2):183-92.
- Dieckmann KP, Anheuser P, Schmidt S, Soyka-Hundt B, Pichlmeier U, Schriefer P, Matthies C, Hartmann M, Ruf CG. Testicular prostheses in patients with testicular cancer – acceptance rate and patient satisfaction. BMC Urol. 2015 Mar 13;15:16.
- Hayon S, Michael J, Coward RM. The modern testicular prosthesis: patient selection and counseling, surgical technique, and outcomes. Asian J Androl. 2020 Jan-Feb;22(1):64-69.
- Data on file at Coloplast Corp. and within the device labeling.
- Robinson R, Tait CD, Clarke NW, Ramani VA. Is it safe to insert a testicular prosthesis at the time of radical orchidectomy for testis cancer: an audit of 904 men undergoing radical orchidectomy. BJU Int. 2016 Feb;117(2):249-52.
- Turek, Paul J. et al. Safety and Effectiveness of a New Saline Filled Testicular Prosthesis. J Urol. 2004; 172:1427 – 143.
PM-29360

