Virtue® Urinary Incontinence Sling
Providing elevation and compression
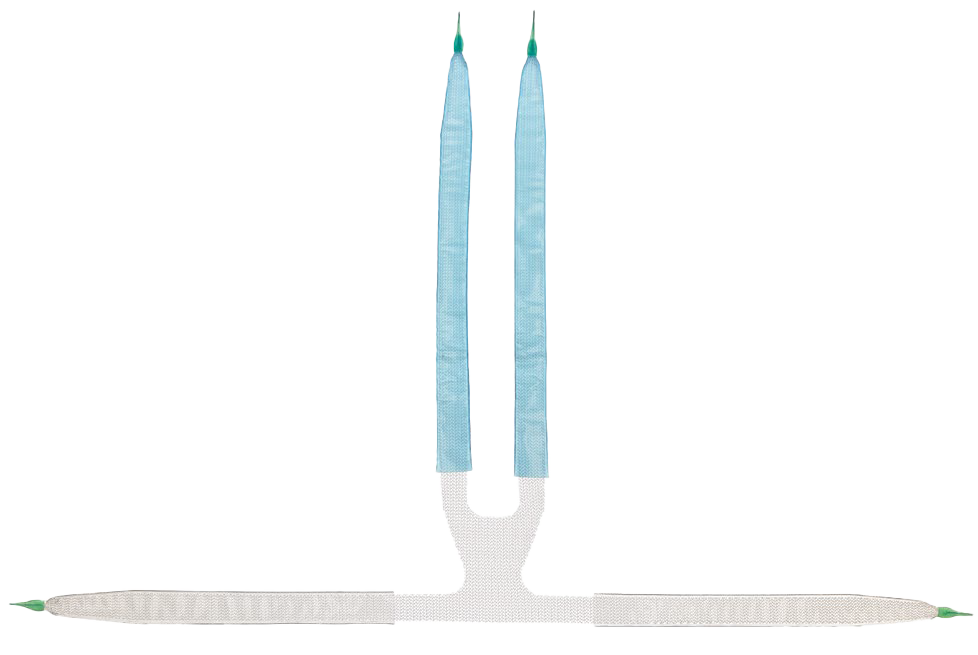
The Coloplast Virtue Male Sling System is designed for stress urinary incontinence (SUI) in male patients. The Virtue Sling is made of 100% monofilament polypropylene.
4-Arm Approach
- Designed to conform to patient’s anatomy
- Ensures consistent placement with quadratic fixation
- Limits sling loosening and proximal or distal movement
Elevation and Compression
- Dual mechanisms of action contribute to patient continence
- Transobturator arms provide proximal relocation through elevation of the bulbous urethra
- Urethral compression provided by prepubic arms
Innovative Placement Technique
- Sub-urethral placement provides support to the bulbous urethra
- Reduced dissection leaves the urethra unexposed
89%
reduction in pad weight at 12 months for moderate range1
Learn about Coloplast solutions
Virtue Male Sling System Brief Statement
Indications
The Coloplast Virtue Male Sling System is an implantable, suburethral support sling indicated for the treatment of male stress urinary incontinence (SUI).
Contraindications
It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the contraindications associated with the use of this product. This product is contraindicated for patients with urinary tract infections or urinary tract obstruction; blood coagulation disorders or prescribed anticoagulation therapy; obstructive uropathy; or, are under the age of 18.
Warnings and Precautions
It is the responsibility of the surgeon to advise the prospective patients or their representatives, prior to surgery, of the possible warnings associated with the use of this product.
Potential Complications
As with all foreign bodies, the Virtue sling system is likely to exacerbate any existing infection. Transitory local irritation at the wound site and a foreign body response may occur. The resulting response could lead to wound dehiscence, extrusion, erosion, inflammation or fistula formation.
The following complications are known to occur with synthetic slings:
- urethral erosion
- infection
- bladder, urethra, vessel and nerve perforation
Known risks of incontinence surgical procedures include extrusion, erosion, infection, sling migration, pain, transient or permanent retention, bladder outlet obstruction, and, continued stress urinary incontinence and persistent or new overactive bladder symptoms.
The information provided is not comprehensive with regard to product risks. For a comprehensive listing of indications, contraindications, warnings, precautions, and adverse events refer to the product’s Instructions for Use. Alternatively, you may contact a Coloplast representative at 1-800-258-3476 and/or visit the company website at www.coloplast.com.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-02084 12.2020
References
- Comiter, Craig et al. The Virtue Sling—A New Quadratic Sling for Male Stress Incontinence—Results of a Multinational Clinical Trial. Urology, Vol. 84, Issue 1, Jul 2014.
PM-29361
